We present a report of a patient with Spinal Muscular Atrophy Type I (SMA) who developed TMA secondary to the infusion of Zolgensma.
Onasemnogene abeparvovec (Zolgensma) is a gene replacement therapy that uses a non-replicating adeno-associated virus (AAV9) to introduce a copy of the SMN1 gene, which is altered in patients with SMA.
Although TMA is a rare complication of Zolgensma, its potential morbidity warrants suspicion.
The patient is a 23-month-old female with Spinal Muscular Atrophy Type 1, tracheostomized and gastrostomized. She was referred to our Hospital for the infusion of Zolgensma. She had received seven previous infusions of Nusinersen (Spinraza), another treatment for SMA.
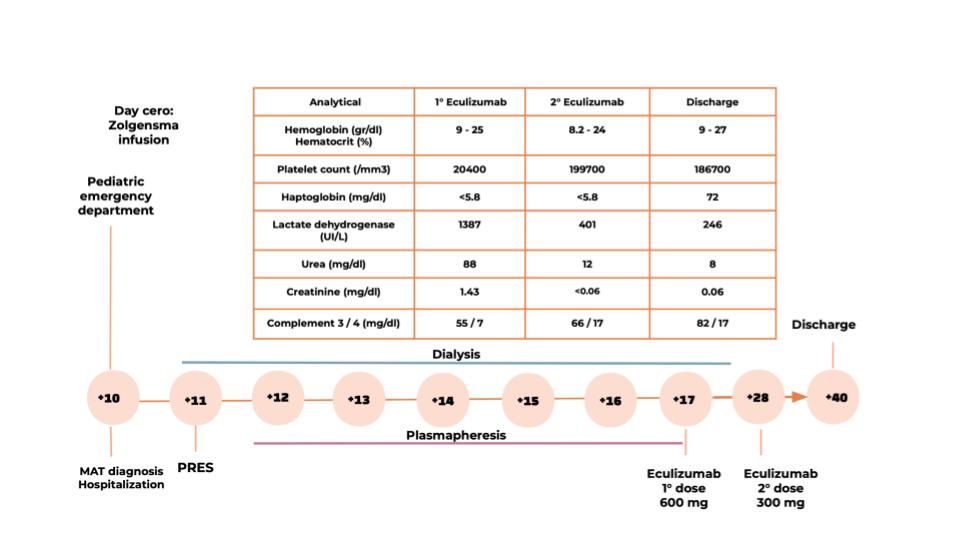
Ten days after the Zolgensma infusion, she presented with vomiting, fever, diarrhea, petechiae and decreased urine output. Laboratory results showed microangiopathic hemolytic anemia (hemoglobin 8.4 mg/dl, lactate dehydrogenase 4400 UI/l, schistocytes in peripheral blood, haptoglobin 7.2 mg/dl), thrombocytopenia (platelet count 45000/mm3), acute kidney injury (urea 237 mg/dl, creatinine 1.96 mg/dl), electrolyte imbalances (plasma sodium 120 meq/lt), low complement levels (C3 64 mg/dl, C4 7 mg/dl), proteinuria, hemoglobinuria and hematuria.
The patient was found to be dehydrated and oliguric. Due to anuria and kidney injury, she began peritoneal dialysis. Within 24 hours she experienced a seizure episode in the context of high blood pressure and a magnetic resonance imaging revealed findings suggestive of posterior reversible encephalopathy syndrome.
Given the neurological involvement, refractory hypertension and low complement levels, the decision was made to perform therapeutic plasma exchange (PE).
After evidence of nonresponse, PE was stopped and complement-inhibiting therapy with Eculizumab was initiated, prescribing two doses according to the infusion protocol.
After ruling out other causes of TMA, such as Shiga toxin-producing Escherichia coli (STEC) infection and thrombotic thrombocytopenic purpura, evidence of complement inappropriately activation was suggest by low C3 serum levels and high levels of C5b9.
Within 96 hours of first Eculizumab infusion, the patient recovered from acute kidney failure, with complete hematologic remission, as shown in the graph.